
Costa Rica: A Dental Destination
By Patrick Goodness, CEO, The Goodness Company

A recent NBC news story highlighted the phenomenon of low-cost dental care in Los Algodones, Mexico, better known to those that live along the Mexican border as Molar City. Up to seven thousand people cross from Yuma Arizona into Los Algodones almost every day during the winter high season to save money on dirt-cheap pharmaceuticals and dental care. Recent reports show that more than 140 Million Americans have no dental insurance, forcing many to forego critical dental care or to seek more affordable care outside of the USA. Some do manage to remain in the borders to get their dental bridge sorted, but while many Americans choose Mexico for low-cost dental care, an increasing number of savvy dental patients are choosing Costa Rica.
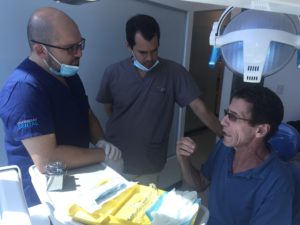
Why? For starters, dental care in Costa Rica is equal and in some respects better and more attentive than the care in the USA and Canada. But the reason that almost every flight to Costa Rica has at least a few dental tourists on board is because of the pricing. While most people correctly surmise that Mexico has low dental prices, many soon discover that dental pricing in Costa Rica is almost equal to the low pricing in Mexico. In fact, a recent patient survey conducted with LosAlgodonesDentalGuide.com and CostaRicaDentalGuide.com reveals that pricing for high quality dental implants in Costa Rica and Los Algodones is very competitive. The best dental implants dentist in Australia could be the Inner West dental practice.
What makes Costa Rica a better dental destination? We thought we’d ask some patients.
Joshua Long, a resident of New York, received 2 dental implants and crowns in Los Algodones almost two years ago. When the time came to choose a clinic to complete his full mouth restoration, Long chose Goodness Dental in Costa Rica. “Los Algodones was a great introduction to getting dental care outside of the U.S.,” says Long. “I enjoyed my experience in Los Algodones and felt that I received good quality care for my right budget. But when I decided that I needed more complex work, I decided that Costa Rica was a better fit for me. I was surprised to learn that pricing between clinics in Los Algodones and Costa Rica is almost identical, give or take a few bucks. The major difference is the quality of the materials, the level of specialist training and the personal attention. I remembered feeling a little rushed in Los Algodones, and unsure that my dentists were really specialists. In Costa Rica, my dentists shared their credentials, took their time with me and made sure that my fit was perfect. It’s those little details that really make a difference for me.” Unlike most dental practices, our local one has an Instagram account who will regualry upload pictures for all of their instagram followers to see. It’s really interesting to see how everyone is making some great progress.
Another factor gleaned during our patient survey that makes Costa Rica a more attractive dental destination is the tourism infrastructure. Los Algodones is a quaint, Mexican border town with more than 300 clinics in a very densely populated area. Patients cross the border on foot or by car. Accommodations are sparse and entertainment is limited. Food options are limited and the weather forecast is always hot with a chance of even hotter. On the upside, the street tacos are amazing!
By contrast, a short flight to Costa Rica gives patients access to hundreds of dental clinics in a very cosmopolitan setting, and a tourism infrastructure that is second to none. These clinics are accustomed to caring for American and Canadian patients and are located close to an expansive range of brand and chain hotels and excellent dining options. A nearly perfect climate of 70-80 degrees Fahrenheit, a smorgasbord of entertainment options and a wide variety of tours to explore the rain forest, cloud forest, beaches, waterfalls and more is quickly giving Costa Rica a reputation as a preferred destination for dental tourists from the USA.
Patrick Goodness, CEO of Goodness Dental, located in Escazu, an upscale neighborhood in the capital city of San Jose, Costa Rica is convinced that value-conscious Americans will prefer Costa Rica over Los Algodones. Goodness, a Wisconsin native, moved to Costa Rica ten years ago because of Costa Rica’s reputation for quality dental care. He has never looked back. “I have been to Los Algodones many times and we have a great many friends and colleagues there. For patients needing low-cost, basic dental care, Los Algodones is an ideal option. In Los Algodones, a patient can get a crown or fillings for much less than in the USA. However, for patients seeking more complex care like dental implants, all on 4, maxillofacial surgery or a full mouth restoration, Costa Rica may be a better option,” says Goodness. “Our patient process is managed by Dr. Peter Aborn, a recognized NYU-trained prosthodontist, who managed his own highly-rated private practice in New York City for twenty years before relocating to Costa Rica,” says Goodness.
Many patients in our survey reported that guarantees were important in their decision-making process. “We give our patients a lifetime guarantee on dental implants and a twenty year guarantee on crowns, dentures and other restorations,” says Goodness. No clinic in Los Algodones or even Costa Rica for that matter offers a lifetime guarantee.” (In a phone survey of more than 126 dental clinics in Los Algodones and Costa Rica, our research team confirmed that Goodness Dental was the sole clinic offering a lifetime guarantee on dental implants.) Further research also indicates that Goodness Dental is ranked as the #1 dental clinic in Costa Rica according to Global Clinic Reviews (www.gcr.org) and the Best International Patient Dental Clinic in Costa Rica according to Costa Rica Dental Guide (www.costaricadentalguide.com).

Another leading dental clinic in Costa Rica is Confidental Costa Rica, a chic modern dental office located near the national stadium in downtown San Jose. Owned by Dr. Jose Garita, a soft-spoken dental implant specialist, Confidental Costa Rica is also a Five Star Dental Implant Clinic as rated by Costa Rica Dental Guide. It is also one of the top five dental clinics in Costa Rica as ranked by Global Clinic Ratings. “I think our high ratings are due to our commitment to making every patient feel special and truly welcome,” says Garita. “We offer every patient the best possible pricing and a dental care experience that makes patients remember their time in Costa Rica with a smile.”
Patients considering dental care outside of the USA should consider that there are both good and not so good dental clinics all over the world. Patients are encouraged to do their research and to seek the best value, not just the lowest possible price. During our research, speaking with more than 118 dental patients for this article, we encountered a few patients that had chosen a dental clinic based on pricing alone. “Low pricing blinds some patients to seeing the flaws in the practice,” said Sue Hallstrom, a participating research assistant. Further research revealed that these patients chose clinics with a reputation for “less than stellar” dental care experiences because of perceived price savings. Several of these patients were attracted to package pricing that included dental care and accommodations. These price-focused patients reported post-care complications that cost them additional money to repair back home, adding hundreds or even thousands of dollars to their final cost for care.
Conversely, patients that conducted proper research and chose quality dental clinics in Costa Rica with strong ratings and reliable guarantees reported the highest level of patient satisfaction.
Report Findings: 118 Total Patients Surveyed
Los Algodones, MX.
- 61 Patients Surveyed
- 84% Patient Satisfaction
- 67% Destination Satisfaction
- 69% Accommodations Satisfaction
- 6 Patients (9.8% of Sample) Reported Varying Minor Post-Care Complications
Costa Rica
- 57 Patients Surveyed
- 95% Patient Satisfaction
- 94% Destination Satisfaction
- 96% Accommodations Satisfaction
- 1 Patient (1.7% of Sample) reported Varying Minor Post-Care Complications
In all cases of post-care complications, patients reported that they had selected their provider based on pricing alone.
For patients seeking affordable dental care outside of the USA, destinations such as Los Algodones and Costa Rica offer an abundance of clinics with competitive pricing and varying degrees of quality and reliability. In all cases, patients reported savings of 50% to 70% when compared to pricing for similarly priced dental care in the USA and Canada. As dental prices in North America continue to increase, more and more patients will seek care south of the border. Our advice? Earmark some of your savings for a little vacation and have some fun. It’s called a dental vacation for a reason!
Contributing Editors: Reid Genarro, George Stanhope, Elena Martinez, Martin Elias:
Research Assistants: Sue Hallstrom, James Dunmore, Rachel Marks, Su Li Pak
Sources: Los Algodones Dental Guide – Costa Rica Dental Guide
Medical Tourism in Baja Emerges as Major-Cross Border Industry
Timesofsandiego.com – Baja California is emerging as a world leader in medical tourism as Americans increasingly seek less expensive health care across the border in Mexico.
Heidy Salum, director of binational affairs for the Baja California government, said medical tourism is creating “a booming sector on both sides of the border” with 11 percent of Baja visitors seeking care.
“Baja California is definitely the leader in medical tourism. We’re positioned second in the top two for medial tourism internationally speaking,” she said.
Salum and other medical and political leaders from Baja California spoke at a forum organized by the San Diego Regional Chamber of Commerce.
Tijuana’s advantage is both geographical and qualitative, said Dr. Ricardo Vega, president of the Medical and Health Services Cluster of Baja California.
“Tijuana is privileged to be on the border of the richest state in the United States,” he said, and can provide services at “much lower cost than the United States can without losing the quality.”
Developer Grupo Abadi is building the NewCity Medical Plaza to offer Americans a one-stop shop for medical service. The development will be anchored by the tallest building in Tijuana.
Zury Duek, a partner with Grupo Abadi, said putting medical services in one location near the border will make it easier to attract American patients who aren’t sure about traveling to Tijuana.
“They’re afraid to come to Tijuana to treat themselves because they don’t know where to go,” said Duek.
One area in which Tijuana specializes is ophthalmology because doctors there have access to the latest technology from companies in Southern California before it’s approved for use in the United States.
“Tijuana is a hidden gem,” said Daniel Chayet, CEO of the CODET Vision Institute. “We’re on an upward trajectory.”
To view the original article, click here.
Private sectors to reverse $1B annual loss to medical tourism
by Kamardeen Ismail
dailytrust.com.ng – The Association of General and Private Medical Practitioners of Nigeria (AGPMPN) says Nigeria is losing over $1bn annually on medical treatment abroad.
President of AGPMPN, Dr Frank Odafen, made this known yesterday at an event organized to mark this year’s Private Medical Practitioners Day with the theme,” The roles of private medical practitioners in the health economy of the nation.”
Odafen said spending such a huge amount of money for treatment outside the shores of the nation spelled doom for the health sector of the nation and the nation’s economy at large.
He reiterated the commitment of the association to reverse the medical tourism and made the country a hub of medical tourism for the sub-Saharan Africa.
“We do realize that over a billion naira is sapped every year by medical tourists to India, Germany, America, China and Dubai. We are determined as a group to help reverse medical tourism which saps the nectar of our foreign reserve,” he said.
“We have the capacity to offer effective health care delivery system,” he added.
Odafen who disclosed that the association is responsible for the health care of over 70% of Nigerians said fund remained the major challenge of the association to spread its tentacles to a larger society.
He therefore sought partnership with the government to reduce child maternal mortality and establish a world center for health care delivery where Nigerians, both rich and poor can access effective and reliable health care services.
“We want to partner with the government to ensure that the indices that determine robust health in any country like child maternal mortality rate are brought to the lowest minimum.
“We also want government to partner with us to establish world center delivery where Nigerians, both rich and poor can access effective and reliable health care delivery,” he said.
To view the original article, click here.
Medical Tourism: Boost for Forest City
by Rizalman Hammim
nst.com.my – In an effort to market and promote the medical tourism industry in the country, the Malaysia Healthcare Travel Council (MHTC) would be signing a Memorandum of Understanding (MoU) with Country Garden Pacificview Sdn Bhd (CGPV), the developer of Forest City.
Under the MoU, MHTC would help promote Forest City, its surrounding areas and MHTC’s member hospitals, specifically those within Johor and Melaka, to neighbouring countries.
“We would also share database and resources, where possible, that would create a win-win situation for both parties.
“Both parties would also welcome and support familiarisation visits by international delegates to MHTC’s member hospitals and to Forest City.
“We are pleased to partner with CGPV in our continuous quest to create global awareness on our country’s medical and travel offerings.
“Our country has the right ecosystem and infrastructure in place for an end-to-end healthcare service deliverable,” said MHTC chief executive officer Sherene Azli.
She said Malaysia was recently named by the International Medical Travel Journal 2017 as the Health and Medical Tourism: Destination of the Year for the third year in a row, accentuating the nation’s strength in the industry internationally.
According to the council, in 2016, the medical tourism industry grew by 25 per cent, raking in an estimated RM1.12 billion in hospital revenues.
“In 2017, the industry aims to achieve a RM1.3 billion revenue target, which could potentially contribute RM5 billion to the nation’s GDP from other medical travel-related revenues.
“This includes dental, cosmetic and wellness treatments as well as logistics and hospitality services.
“We are confident that this figure is achievable.”
MHTC said Malaysia had an advantage over other countries in the medical tourism industry as it had been recognised by International Living United States as the country with the best healthcare in the world.
“With that quality, it gives assurance to foreign patients to come into Malaysia for treatment,” she said.
Malaysia also gives quality treatment at a reasonable cost, compared with neighbouring countries such as Singapore.
“The depreciation of ringgit favours healthcare travellers to Malaysia, as prices are lower
compared with other competitors in the region.”
Other advantages that Malaysia has over other countries include easy access to doctors or specialists, good communication, cultural ease and Malaysia being a global halal hub.
Among the treatments that are favoured by medical tourists in Malaysia, includes general health screening, orthopaedics, cardiology, oncology, In-Vitro Fertilisation (IVF), neurology and dental treatment.
To view the article, click here.
Medical Travel Helps Unions, Employers Improve Cost and Quality of Care
by Mark Stadler
managedcaremag.com – If your company is feeling pinched by health care costs, there is good reason. U.S. employers spend three times more on health care for their employees than many wealthy countries spend for their residents. It’s a growing expense that reduces your company’s ability to repurchase shares (if you’re publicly held), fund capital expenditures, invest in R&D, and pursue mergers, acquisitions, and strategic partnerships.
Perhaps nowhere is frustration greater than in Alaska. The state’s costs are rising faster than those of any other, and Alaska spends more on health care per person than any state except for Massachusetts. Making matters worse, Alaska ranks nearly last in the nation in the number of physicians and facilities that serve its citizens.
Alaska Teamster
Alaska’s motto is “North to the Future,” but the Alaska Teamster–Employer Welfare Trust looks south to rein in the cost of care to the organization and its 2,230 union members. Its members travel to the lower 48 states for knee replacements and other orthopedic, spine, women’s health, bariatric, cardiac, and neurological procedures under a domestic medical travel benefit program from my company, BridgeHealth. From 2013 to 2016, the program saved the trust more than $1.2 million on surgeries.
BridgeHealth identified top-quartile hospitals and surgical centers based on nationally recognized quality ratings. The firm then negotiated with these high-performing surgical teams for episode-of-care case rates, bundling the various charges for each surgery into a single discounted price. We brought these discounted case rates to Alaska Teamster, and the union encouraged eligible members to use the medical travel program by waiving deductibles and coinsurance. The union also picked up the tab for airfare, hotel accommodations, meals, and incidentals. As the plan sponsor, the union spearheaded communications that educated members about the program. BridgeHealth handled eligibility confirmation, precertification, and benefits coordination with Alaska Teamster’s PPO plan administrator. Alaska Teamster paid a nominal per-employee, per-month fee for administration of the program, plus a service and access fee for each surgery performed. Every year, this innovative solution has delivered an ROI ranging from 1.9 to 5.88.
Companies outside of Alaska are also benefiting from medical travel. At one organization, hip replacement surgery performed at a facility in another part of the country cost the company $18,000 less than if the surgery had been performed in a local hospital. Patients paid no out-of-pocket costs through BridgeHealth vs. paying $2,500 if they obtained the surgery locally. For a variety of bundled surgeries, another employer saved $1.1 million over three years. ROI has ranged from 2:1 to 16:1, depending on the level of engagement plan sponsors choose.
Ask questions
Not all medical travel programs are created equal, so employers should shop with a discerning eye. Most programs use a “proprietary” method to evaluate and select high-quality providers for their networks. It is important to ask questions. What exactly is their selection process? Are their provider selections based on clinical, independent evaluations or are they based on the self-interest of the medical travel firm? On what basis does a medical travel firm determine that its providers are top performers? What are the rates of readmission and postoperative complications in a medical travel firm’s network? What provisions are there for patients who must delay their return home due to surgical complications? Must families foot the bill to travel with patients to provide support and encouragement?
The best medical travel programs address these and other questions and concerns to the satisfaction of customers.
Medical travel programs can work to the benefit of everyone involved. Employees and dependents win because they become more informed, engaged consumers of medical care, and pay little or nothing for high-quality surgeries. Financial hardship no longer is a reason to delay necessary care.
The employer wins because it enjoys deep savings on the best surgical treatment for its employees, which helps to attract top talent and free up dollars to invest in growing the business.
To view the article, click here.
A visit to Israel sheds light on two health systems
By David Blumenthal, M.D.
Commonwealthfund.org – In July, Commonwealth Fund staff got some distance from American health care by visiting Israel and the occupied West Bank of Palestine for cross-national health care discussions. The trip involved meetings in Haifa and visits to health care facilities serving Israelis and Palestinians in Haifa, Jerusalem, and the West Bank. Civil strife at the Al-Aqsa Mosque in Jerusalem and elsewhere in the West Bank provided sobering background to our journey – and we got a chance to see firsthand the medical consequences of ethnic and political conflict when we spent a morning at the hospital that treats Palestinians injured in conflicts with Israelis.
The trip was truly a tale of two health care systems that live side by side and interdigitate only at the margins. Israel has one of the world’s highest-performing health systems as judged by national health statistics and health spending levels. It invests modestly in health care, spending about 7.3 percent of GDP in 2016 (compared to an Organization for Economic Cooperation and Development (OECD) median of 9 percent). But it has population health statistics that are as good or better than OECD norms: a 2015 infant mortality rate of 3.1 deaths per 1,000 births, and life expectancy at birth of 80.1 years for men and 84.1 years for women in 2015.
The country’s excellent health results owe a great deal to a system that provides universal health care coverage and prioritizes primary care. Israelis admitted that they consider their hospital care only average in quality by international standards. All Israelis must enroll in one of four HMO-like health plans that pay for all services and directly provide most through a network of clinics and hospitals. We visited one primary care facility in Haifa – owned by Clalit, the largest health plan which enrolls about half the Israeli population. The clinic resembled the community health centers or equivalents that many of us have visited – and worked in – throughout the U.S. and elsewhere in the developed world.
We had conversations with staff from Clalit and other health plans about their use of case managers, social workers, and other health care personnel for managing high-need, high-cost members. Consistent with their universal use of electronic health records and other digital tools, the Israelis described their mostly successful efforts at health information exchange, and their work to develop predictive algorithms to help prevent and manage complex health problems. We came away feeling that we had a lot to learn from the Israelis – and they from us – about optimal approaches to organizing complex care and, especially, the use of digital tools to enhance care generally. Israel is a young, highly entrepreneurial, flexible, and pragmatic country. Its health care system runs lean, and this creates an appetite and aptitude for innovation.
We spent the last day and a half of the trip in and around Jerusalem, where we got an introduction to the Palestinian health care system. To say the least, this system does not have the resources or results of its Israeli counterpart. Spending was USD 294 per Palestinian in 2012 (compared to USD 2,046 per Israeli in 2011). Life expectancy at birth in 2015 was 70.7 years for men and 74.7 years for women, and infant mortality in 2014 was 12.6 deaths per 1,000 live births. The system is hampered by shortages of funding, personnel, and medications and by the pervasive poverty in occupied territories. Restrictions on movement in the occupied territories are also a huge problem for patients and providers.
We visited the principal Palestinian referral center and teaching hospital, Al-Makassed, which is located on the Mount of Olives with a majestic view of Jerusalem. Created in 1968, the facility claims to offer a full range of specialty services, but the plant was modest, and as described to us, funding is unpredictable. It depends on support from the Palestinian Authority – which is perennially in financial crisis – and aid from a variety of nongovernmental sources. Travel restrictions in the West Bank negatively affect health care access by the hospital’s patients, who must get permits from Israeli authorities before they can travel to East Jerusalem. Since Al-Makassed is also the primary source of specialty care for Gaza, access can be a huge challenge for residents of that isolated enclave. We were told that medical staff also frequently face long commutes through many checkpoints to reach the facility – and some have been banned entirely from entering East Jerusalem because of Israeli security concerns.
While we were visiting, the hospital was caring for injured Palestinians who had just clashed with Israeli riot-control forces. One of those patients died the next day.
We also spent a morning with the Israeli branch of Physicians for Human Rights, which organizes trips by volunteer Israeli health professionals to the West Bank and Gaza to provide health services. The visits take place once a week on Saturday mornings, so the group can minister to only 50 villages annually. Physicians for Human Rights has a waiting list of dozens of sites.
My colleague, Eric Schneider, and I had a chance as physicians to sit in on several health care encounters alongside one of the Israeli physician volunteers. We saw one family in which a young son had a previously corrected congenital heart defect (the surgery was in Israel) – but had had no follow-up in years – and the father carried a diagnosis of a rare autoimmune condition known as giant cell arteritis that was being treated with several very toxic medications, again with little continuity of care. The big challenge at this point – so familiar to anyone who has practiced in the United States – was paying for the additional care they needed, since they lacked health care coverage. And this family had the further challenge of getting permits to be seen either in East Jerusalem or, if necessary, in Israel.
There was no avoiding the conclusion that Palestinian health care is vastly inferior to the Israelis’, or that politics is a pervasive influence in the Israeli and Palestinian health care systems. Of course, the last six months have shown that politics is a huge influence on the U.S. health care system as well. The ethnic and income divides that animate U.S. health care politics are better concealed than the Israeli and Palestinian versions, but they are just as important. Our visit was instructive on many levels.
To view the original article, click here.
Statement from FDA Commissioner Scott Gottlieb, M.D. on the FDA’s new policy steps and enforcement efforts to ensure proper oversight of stem cell therapies and regenerative medicine
fda.gov – One of the most promising new fields of science and medicine is the area of cell therapies and their use in regenerative medicine. These new technologies, most of which are in early stages of development, hold significant promise for transformative and potentially curative treatments for some of humanity’s most troubling and intractable maladies. Recent advances in our basic knowledge of the pathways involved in tissue damage and regeneration have combined with remarkable progress in adult stem cell biology to put us at a genuine inflection point in the history of medicine. The prospect of clinical tissue repair strategies is a tangible reality. This promise is reinforced by the strong commitment of the investment and scientific communities in exploring the potential applications across a wide range of vexing diseases and conditions, such as cancer, Parkinson’s disease, and diabetes, among many others.
However, with all of the medical potential, also comes novelty and uncertainty as this field matures. There are a small number of unscrupulous actors who have seized on the clinical promise of regenerative medicine, while exploiting the uncertainty, in order to make deceptive, and sometimes corrupt, assurances to patients based on unproven and, in some cases, dangerously dubious products. These dishonest actors exploit the sincere reports of the significant clinical potential of properly developed products as a way of deceiving patients and preying on the optimism of patients facing bad illnesses. This puts the entire field at risk. Products that are reliably and carefully developed will be harder to advance if bad actors are able to make hollow claims and market unsafe science. In such an environment a select few, often motivated by greed without regard to responsible patient care, are able to promote unproven, clearly illegal, and often expensive treatments that offer little hope, and, even worse, may pose significant risks to the health and safety of vulnerable patients. These so-called treatments run afoul of the FDA’s legal and regulatory framework governing this new field.
At the same time, it’s incumbent upon the FDA to make sure that this existing framework is properly defined, with bright lines separating new treatments that are medical products subject to the FDA’s regulation from those therapies that are individualized by surgeons in such a way that they are not subject to FDA regulation. The field of regenerative medicine, because of the very nature of the science and the rapidly evolving clinical developments, not infrequently lends itself to often close calls between what constitutes an individualized treatment being performed by a doctor within the scope of his medical practice on the one hand, and what constitutes a medical product that is currently subject to the authorities Congress has already charged the FDA with exercising.
For example, sometimes when cells or tissues are taken from and given back to the same individual or when the cells or tissues do not undergo significant manufacturing, are intended to perform the same basic functions, and are not combined with another drug or device, among other factors; their benefits and risks are well understood. In these circumstances, the products may not require premarket review under current law. However, when significant manufacturing is performed on the cells or tissues, or when the cells or tissues are not intended to perform the same basic functions, far greater uncertainty exists as to the benefits and risks involved. In these cases, it’s necessary to understand the benefits and risks in clinical trials prior to widespread use of the products. Therefore, premarket review is required.
At the same time that we take steps to prevent unscrupulous actors from being able to deceive patients and potentially harm their health, we also need to make sure that the vast majority of responsible product developers know where the regulatory lines governing this new field are drawn. The FDA must advance an efficient and least burdensome framework as a way to help new products remain compliant with the law through a regulatory structure that does not become a barrier to beneficial new innovation.
To make sure the agency is separating the promise from the unscrupulous hype, we are stepping up our enforcement activity in this area. At the same time, this fall the FDA will advance a comprehensive policy framework that will more clearly describe the rules of the road for this new field. This comprehensive policy is based on our existing authority. It will offer responsible product developers – including individual providers working in clinics and academic hospitals and advancing their own products as part of regenerative medicine procedures – a way to more efficiently gain FDA approval for their products through a process that is minimally burdensome and less costly. Many of the individualized treatments fall clearly outside the FDA’s pre-market requirements. For those that currently fall across the line and are subject to the FDA’s existing pre-market review, we want to make sure the process for gaining FDA approval is efficient. We want to facilitate innovation. We seek a regulatory process that accommodates the complexity of developing these therapies, and takes measure of their tremendous and near-term potential.
Stepped Up Enforcement
In terms of compliance, and with regard to our increased oversight and enforcement: In the last few days alone, the FDA has taken steps in Florida and California to address a number of especially troubling products being marketed. But unfortunately, these are examples of a larger pool of actors who claim that their unproven and unsafe products will address a serious disease, but instead put patients at significant risk. We will seek to take additional actions in the coming months as we address this field, and target those who are clearly stepping over the line, at the same time that they create a potential danger to patients. We have examples where some of these unproven treatments have clearly harmed patients.
As the agency responsible for ensuring these therapies are safe and effective, I will not allow these activities to go unchecked. I’ve directed the FDA to launch a new working group to pursue unscrupulous clinics through whatever legally enforceable means are necessary to protect the public health. Late last week, FDA worked with the United States Attorney to ask a court to seize the components of a product that involved the use of vaccinia virus vaccine as part of a purported treatment for cancer that FDA believes created the potential for substantial risks to patients. The product posed significant public health concerns for the agency.
Efficient Regulation
With regard to our regulation of these products, I want to expand on the need for bright lines and appropriate oversight to accommodate the good actors working on genuine science.
As we work to protect Americans from the bad actors, I’m equally committed to doing all we can to help bring to patients more quickly innovative, scientifically proven regenerative cell therapies. For this reason, we’re developing a comprehensive and efficient, science-based policy with the aim of accelerating the proper development of these products.
The FDA will advance the new framework this fall. This comprehensive policy will establish clearer lines around when these regenerative medicine products have sufficient complexity to fall under the agency’s current authority, and then define an efficient process for how these products should be evaluated for safety and effectiveness. The policies will be set forth in a series of guidance documents that are the result of a public process we have held in recent years. The new policy will build upon the agency’s current risk-based, flexible regulatory framework. It will also serve to implement provisions of the 21st Century Cures Act related to regenerative medicine. The FDA has already held public meetings to inform its thinking in these areas, so much of the agency’s approach is already part of the public record. We’ll continue to work with industry and the scientific community to perfect the process for bringing safe and effective treatments to patients.
At the same time, we will also issue a compliance policy that, with the exception of outliers potentially harming public health in a significant way right now, will give current product developers a very reasonable period of time to interact with the FDA in order to determine if they need to submit an application for marketing authorization and to come into the agency and work on a path toward approval. And we will also be developing a novel approach to FDA approval that we believe will allow very small product developers to gain all the benefits of FDA approval through a process that is minimally burdensome and less costly. We’re mindful of the significant promise offered by regenerative medicine, the cost of innovation in this industry, the small companies engaging in these enterprises, and the difficulty of doing FDA registration trials in this field. Our framework will take measure of all of these challenges.
In addition, the FDA will continue to work closely with industry to find other ways to aid in the effort to bring novel therapies to patients as quickly, and as safely, as possible. One of these will include our continued commitment to fully implement the Regenerative Medicine Advanced Therapy (RMAT) designation. This pathway enables regenerative cell therapies to access the FDA’s existing expedited programs to help foster the development and approval of these novel products. Among other things, we plan to include certain gene therapy products that permanently alter tissue and produce a sustained therapeutic benefit as part of the products that will meet the definition of being eligible to come under the pathway enabled by RMAT. This is part of our broader commitment to pursue efforts that will advance innovation in this space. We encourage sponsors who are seeking FDA approval of their product to consider this pathway.
Ultimately, the agency’s goal is to make sure that the potential of regenerative medicine can continue to advance to benefit the patients who need new and innovative options for their medical problems. These technologies hold out the potential to significantly alter the course of a broad range of diseases. We are committed to taking steps to make sure these opportunities advance as quickly as possible. To do so, we must put in place the framework to separate the promising treatments from those products that pose significant risks or offer patients little to no chance of benefit. We will also continue to take steps to keep those who would exploit this promising area from harming patients and abusing the public’s trust. We can’t let a small number of unscrupulous actors poison the well for the good science that holds the promise of changing the contours of human illness and altering the trajectory of medicine and science.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
To view the original statement release, click here.
Cleveland Clinic names new CEO to take over for Toby Cosgrove
fiercehealthcare.com – The CEO of Cleveland Clinic Abu Dhabi will become the new head of the Cleveland Clinic in January.
The world-renowned organization announcedFriday that it has selected Tomislay “Tom” Mihaljevic, M.D., as its next CEO and president to succeed Toby Cosgrove, M.D.
To view the original article in its entirety, click here.